Chemical Vapor Transport is a novel method of synthesis that can be used when other methods, such as flux growth, are not possible, or as a way to purify metals. Such examples of syntheses like this include Hf2Te2P where Phosphorous readily sublimes at slightly above room temperature or in cases where a rare phase of the material is desired. It exists in nature mostly in regions such as volcanoes, which supply large amounts of high temperature gas flow. Volcanoes can grow Hematite using HCl gas as a transport agent in nature. [1]
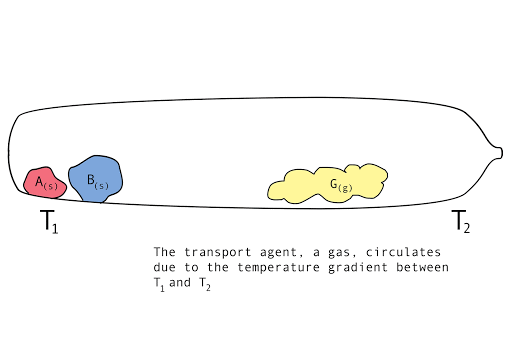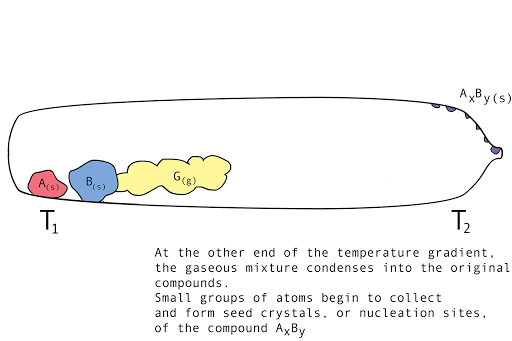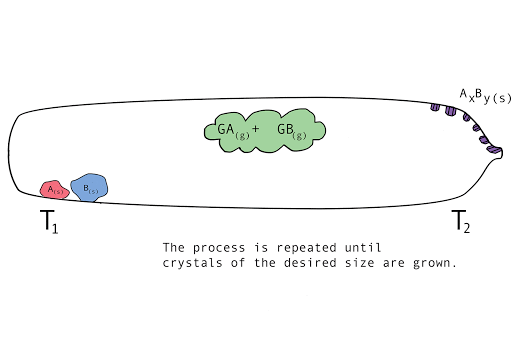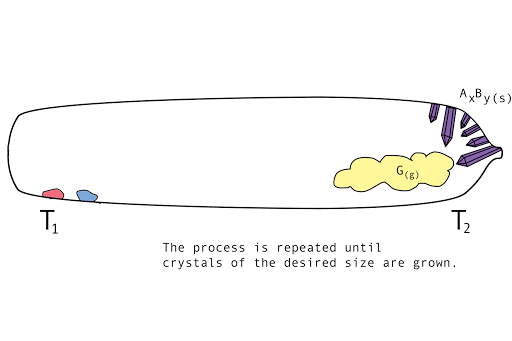
Figure 1. A diagram showing the vapor transport of compounds A and B with a continuous stream of transport agent, gas G.
Chemical vapor transport reactions are characterized by a temperature gradient, a forward reaction, gas flow, and a back reaction. These features characterize the kinetics of the reaction, affecting the mass transport rate. At one end, called the source, the parent compounds are mixed with another compound or mixture, called the transport agent. At the other end, called the sink, is the crystal growth region. There are three stages to a chemical vapor transport reaction.
- Forward reaction: parent compounds and transport agent mix at T1. They form a volatile intermediary compound.
- Gas circulation: This gaseous mixture flows, due to the temperature gradient, over to T2.
- Back reaction: At T2, the synthesis reverts back to the original materials, depositing first small seed crystals called nucleation sites, then depositing the molecules of the parent compounds in a regular, crystalline pattern (crystal growth). The transport agent flows back to T1.
For exothermic reactions, the gas flows from the cooler region to the hotter region of the ampule. For endothermic reactions, the the gas flows from the hotter regions to the cooler regions of the ampule.
Good transport agents are typically halogens and halogen compounds due to their reactivity with the compound. [2]
Factors that affect crystal growth [3]:
- The Stoichiometry of the reaction will affect the velocity of the gas and kinetics of the reaction
- The Flow Rate affects the kinetics of the reaction as well as the size of the crystals
- The temperature gradient as well as the length of the ampule affect the rate of mass transport in the reaction as this changes the partial pressure of the gas in the ampule [1]
Examples of Vapor Transport Syntheses:
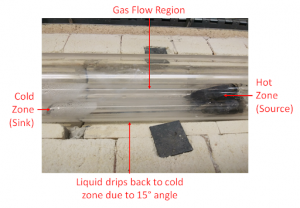
Figure 2. A diagram showing the experimental setup of a FeSe synthesis

Figure 3. A timelapse of the growth of Fe1.1Se0.863S0.137

Figure 4. Synthesized crystals of Hf2Te2P
References:
[1] Binnewies, M., Glaum, R., Schmidt, M. and Schmidt, P. (2013), Chemical Vapor Transport Reactions – A Historical Review . Z. anorg. allg. Chem., 639: 219-229. doi:10.1002/zaac.20130004[2] Binnewies, M., Schmidt, M. and Schmidt, P. (2017), Chemical Vapor Transport Reactions – Arguments for Choosing a Suitable Transport Agent. Z. Anorg. Allg. Chem., 643: 1295-1311. doi:10.1002/zaac.201700055
[3] Schmidt, Peer, et al. “Chemical Vapor Transport Reactions–Methods, Materials, Modeling.” Advanced Topics on Crystal Growth, IntechOpen, 2013, doi:10.5772/55547.